Relating atomic energy, radius and electronegativity.
Introduction
The challenge of estimating atomic radii at high pressureNuclear radii and electronegativity are frequently quintessential for how science rationalized.1,2 The historical backdrop of measuring the extents of particles under surrounding conditions incorporates a huge group of work, reaching out over the final remaining one and a half-hundreds of years (for a non-thorough synopsis of this set of experiences see ref. 3 and 4). One early inspiration for accomplishing nuclear and ionic sizes was to assist with understanding X-beam diffraction designs as far precious stone structures,5,6 one more to give a justification to metallization.7-9 Today, different meanings of nuclear radii with notable purposes exists, including, e.g., ionic,10-12 covalent,6,13-17 and vdW radii.3,18-21 Electronegativity is a comparatively very much concentrated on idea that can be characterized in numerous ways (see, e.g., ref. 22-29 and references in that).Relating atomic energy, radius and electronegativity.
The nearer the electrons are the core, the more firmly they bound, hence expanding the electronegativity of the molecule. Pitzer called attention to the occasional conduct in the two nuclear properties long ago30 and numerous others have depended on various meanings of nuclear radii (normally covalent radii) and electrostatic connections to characterize sizes of electronegativity.24,31-44.Relating atomic energy, radius and electronegativity.
electronegativity
There exist various systems through which electronegativity may connected with radii under pressure. Garza et al.45 and Chattaraj and coworkers46,47 have, for instance, depended on reasonable thickness useful hypothesis to assess the electronegativity for a choice of iotas compacted by impervious circular depressions. In a connected work, Sen et al. have determined the basic distance across at which round imprisonment causes ionization of some atoms.48 In this work, we depend on two reconsidered sizes of nuclear vdW radii3 and electronegativity29 which have been reached out to high tension circumstances (0-300 GPa).4,49
The size of electronegativity utilized here is enlivened by crafted by Allen26 and is characterized as the normal electron restricting energy as T → 0 K.29,50 This meaning of electronegativity lays out an association with the complete energy of a framework through an energy decay analysis:51
ΔE = −nΔ![[small chi, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d8.gif) − ΔEee + ΔENN,
− ΔEee + ΔENN,
where ΔE is the adjustment of complete energy over a compound or actual change, n is the all out number of electrons, Δ[small chi, Greek, macron] is the adjustment of electronegativity (here characterized as the normal electron restricting energy), while ΔEee and ΔENN are changes in the electrostatic repugnance between electrons (affected by trade and relationship impacts) and cores, separately.
The challenge of estimating atomic radii.
Pressure is a naturally visible discernible, characterized with regards to a gathering of molecules. We can, consequently, on a basic level, relate the volume V of eqn (2) to a typical nuclear volume. Nuclear volumes can, and have been, estimated as a component of strain utilizing different exploratory strategies (see, e.g., ref. 52-54). We note that Youthful has given a schematic outline of nuclear volumes at chose higher tensions got by a combination of examination and computation on consolidated phases.53,54 notwithstanding an abundance of condition of-state information, a test emerges when one attempts to separate nuclear radii from volumes of genuine materials.Relating atomic energy, radius and electronegativity.
Likewise, assuming we take a gander at nuclear volumes of heavier components, the radii removed will compare to metallically reinforced radii, not vdW radii, and will besides rely upon (translucent or fluid) structure. Approximations of vdW radii under tension have gotten with regards to the actuation volume of a few natural responses, however not methodicallly revealed (see, e.g., ref. 55).
The test of non-fortified/vdW radii can tackled computationally by considering single molecules compacted by a homogeneous non-responding environment.4,49 The properties – radii, electronegativity as well as ground state electron setups – of such packed iotas not entirely set in stone in the tension territory from 0 to 300 GPa through maximum capacity relativistic thickness utilitarian hypothesis estimations joined with the Outrageous Strain Polarizable Continuum Model (XP-PCM).4,49,57,58 We stress that by utilizing this technique we deliberately reject the impacts of both gem design and compound holding. The extents of the iotas are in our model simply an outcome of isotropic non-receptive pressure. Such registered non-reinforced high-pressure radii are in fantastic concurrence with exploratory pressure isotherms for respectable gas components, when trial radii are characterized utilizing eqn (3).
Results and discussion
We will in what follows take a gander at the development of electronegativity and range figured for a chose mix of iotas.database.59Fig. 1 shows a selection of our information and looks at the difference in the non-fortified sweep and electronegativity of Mg and Al with pressure.
The top piece of Fig.
suggests areas of strength for an electronegativity on nuclear sweep – when individual molecules packed their size decreases alongside their electronegativity. This reliance currently evaluated for any iota inside our meaning of these properties.4,49 We note that, since we consider pressure of non-cooperating molecules, ends drawn from our information can in cases show up in conflict with related work where electronegativity is rather characterized using covalent radii or warms of response, for example, in crafted by Batsanov.41,60 The errors happen to a limited extent on the grounds that covalent radii may both increment and diminishing in specific tension ranges.61 conversely, nuclear volumes of non-reinforced particles (as well as fortified components) monotonically decline with pressure. The base piece of Fig. 1 demonstrates the way that patterns in the progressions of radii and electronegativity can be inverse in an overall correlation between particles.
Relating atomic radius, electronegativity and energy
Fig. 2 shows the difference in the non-reinforced span and electronegativity of Fe with pressure comparative with Si. These molecules are two of the main constituents of the World’s covering, mantle (p < 140 GPa), and center (p < 360 GPa), and decided here to delineate exactly the way that drastically various we can anticipate that science should be at various thermodynamic circumstances. The sharp discontinuities anticipated in the two properties at 30 and 144 GPa harmonize with advances of the ground state electronic setup of the Fe molecule. Such advances, exemplified for Fe in Fig. 2, are normal to numerous antacid, basic earth, change metal, and f-block molecules, and are notable from both hypothesis (e.g., ref. 49, 62 and 63) and analyze (e.g., ref. 53 and 64). Ground state configurational changes in non-reinforced particles (and numerous materials) are isobaric processes,
-
Example
Instances of the connection between nuclear range and electronegativity. Changes in [small chi, Greek, macron] (left) and range (right) of Fe during non-responsive pressure. The information shown comparative with Si. Green circles and red squares show two tensions, both at which the Fe iota can have both of two radii, two electronegativities and two energies. The two vertical drops concur with [Ar]4s23d6 (S = 2) → [Ar]4s13d7 (S = 2) and [Ar]4s13d7 → [Ar]3d8 (S = 1) ground state configurational changes in the Fe iota. Slight ruggedness in the information for the radii is a computational relic emerging from extrapolation from a limited number of pressure estimations. The strain advancement of radii and electronegativity for different iotas can pictured with the AUP database.
Eqn (6) advises us to expect diminishes in electronegativity when the sweep of an iota diminished under consistent tension. Electronegativity likewise diminishes as electron aversion, measured by the ΔEee-term, increments. We remind that eqn (6) has determined for pressure of non-responsive iotas. Contentions in light of this situation are accordingly not really consistently pertinent to different circumstances, for example, volume changes evaluated through, e.g., trial conditions of state. Eqn (6) by the by assists us with understanding how decrease of the oxidation condition of a molecule under encompassing circumstances (where the temperature is low and p ≈ 0) prompts a decline in electronegativity (this occurs as ΔEee > 0 for electron connection).
Interpreting changes in radii in terms of energy
Eqn (1) moreover advises us that the diminishing in electronegativity, or the typical orbital destabilization, in such iotas upon pressure doesn’t approach the corresponding change in the complete energy. The connection between changes in energy and electronegativity incorporates a duplication by the quantity of electrons n, and the deduction of the non-inconsequentially measurable electron communications depicted by the ΔEee-term.
What electronic energy can credited to the spiral constrictions displayed in Fig. 2 (and the numerous others detailed in ref. 4)? Eqn (5) shows that this relies upon both the progress pressure and the range of the particle.
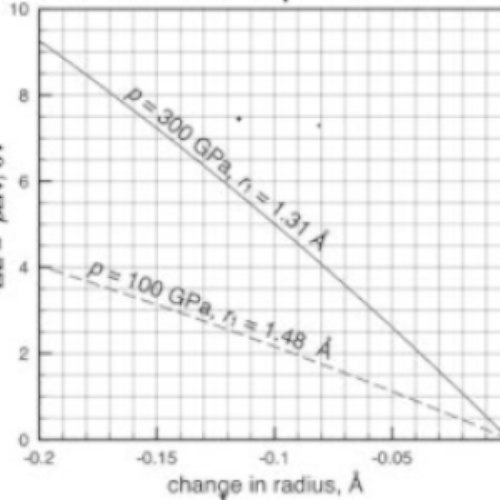
In our illustration of Fe, the iota anticipated to get its sweep from 1.68 Å to 1.63 Å at a tension of 30 GPa. For this situation, the comparing energy change, ΔE, viz.eqn (5), rises to 0.43 eV (Fig. 3). In the subsequent progress, the Fe particle recoils by a comparative size from 1.38 Å to 1.31 Å. As a result of a higher strain at the subsequent progress, the relating energy change is, nonetheless, significantly bigger, 1.95 eV (Fig. 3).
Biggest impact
The biggest impact of a s → d change in the d-block anticipated for Cr, where a compression by 0.08 Å at 208 GPa converts into an electronic energy increment of 3.0 eV with Δ[small chi, Greek, macron] = 5.8 eV e−1. One more model is Ce, which anticipated to go through a little outspread constriction by 0.02 Å at 270 GPa. The Ce constriction means a 1.0 eV change in complete energy. Be that as it may, the anticipated comparing decline in electronegativity of Ce at this change pressure is a lot bigger at 5.5 eV e−1. The distinction between nuclear size and electronegativity is perceptibly bigger for the f-block particles, a reality we quality to additional significant changes in their electron communications (the ΔEee-term in eqn (6)) during isobaric changes, contrasted with the lighter components.Relating atomic energy, radius and electronegativity.
Conclusions
The image that rises up out of our examination is seemingly one in which nuclear span and electronegativity walk connected at the hip; the two properties decline with pressure comparative with surrounding conditions. Nonetheless, relative contrasts in these nuclear properties may both increment or diminishing with an irritation, for example, compression.4,49
Following the computation of sweep and electronegativity as a component of strain in past work,4,49 we here determine eqn (6) that interfaces the two amounts in isobaric changes of non-reinforced molecules. The system we have illustrated can make ready for a more broad comprehension of these focal synthetic idea with more extensive ramifications in science and materials science. Eventually, these nuclear properties, regardless of how characterized or measured, are only approximations and advisers for the way of behaving of genuinely reinforced materials. Point by point investigations and thought of electronic design will continuously be fundamental for quantitative assessments of, e.g., security strength and polarity.66,67Relating atomic energy, radius and electronegativity.